Introduction
Periodontal Disease (PD) is a group of inflammatory diseases that affecting the supporting tissues of the teeth. Based on the theory of “focal infection” which emerged at the beginning of the twentieth century, many studies have investigated a possible role for PD as a risk factor for systemic conditions over the past two decades[1], including cardiovascular diseases[2], diabetes[3], adverse pregnancy outcome[4], osteoporosis[5], rheumatoid arthritis[6], and Chronic obstructive pulmonary disease (COPD).[7],[8]
COPD is also an inflammatory disease characterized by the progressive deterioration of pulmonary function and increasing airway obstruction, includes chronic bronchitis and emphysema[9]. In line with the relationship between the anatomical position of oral cavity and pulmonary infection, oral bacteria can be easily carried into the lung and cause infection[10]. In addition, PD and COPD share the same risk factors, including smoking, age, obesity, socioeconomic status, and living conditions[1]. These data strongly suggest that PD may be a risk factor for COPD and that oral bacteria may play a key role in its progression.
Associations between respiratory diseases and oral health in community-dwelling populations were first determined by analysis of the National Health and Nutrition Examination Survey I data set.[11]. Logistic regression analysis revealed that poor oral hygiene and smoking status were statistically associated with chronic respiratory disease. The study of Hayes et al[12] found periodontitis, measured as alveolar bone loss assessed from periapical radiographs, to be an independent risk factor for COPD in adult males enrolled in the VA Normative Aging Study. Associations between respiratory diseases and oral health incommunity-dwelling populations were first determined by analysis of the National Health and Nutrition Examination survey.
Several biologically plausible mechanisms have been put forth to explain how periodontitis can lead to respiratory disease. Salivary enzyme activity is increased in periodontitis and can promote the adhesion of pathogenic bacteria to the oral surfaces, thereby altering oropharyngeal colonization patterns.[13],[14] In addition, oral bacteria involved in periodontitis can stimulate oral tissues and periodontium to release cytokines, which are proteins involved in cellular interactions and immune responses. These cytokines can promote adhesion of respiratory pathogens to mucosal surfaces, thereby leading to oropharyngeal colonization.
Periodontitis may also affect pathogen adhesion to respiratory epithelium. In vitro studies indicate that the presence of Streptococcus gordonii, a key bacteria in dental plaque formation, enhances the ability of pathogens such as H. influenzae to adhere to respiratory epithelial cells.[17] In response to bacterial adhesion, respiratory epithelial cells may release cytokines and attract neutrophils, which in turn release proteolytic enzymes that damage the epithelium and increase its susceptibility to infection.[15],[16] In addition, cytokines released from inflamed periodontal tissues may enter the respiratory tract in aspirated saliva, triggering the same sequence of events, including neutrophil recruitment, epithelial damage, and infection.[16]
Therefore, an attempt to find out the relation between periodontal diseases and COPD has been made.
Materials And Methods
In this study, 25 patients were selected on following selection criteria from the hospitalised patients from the Department of General Medicine from Rohilakhand Medical college Bareilly. The patients were in the age more than 20 years with at least 6 natural teeth present with exacerbation of COPD (case group) .Patients with no past or present history of COPD were included in the control groupand were selected from institute of dental sciences bareilly.Exclusion criteria for control groupwas edentulous patients, if patients had undergone periodontal therapy for last 6 months, patients on medications (antibiotics) known to influence the periodontal tissues other than COPD, patients with any other systemic diseases other than COPD, patients in intensive care unit.Exclusion criteria for case group was patients with any systemic diseases, patients under any medication for last six months, patients under any medication.
The physician made the diagnosis of respiratory diseases and non-respiratory diseases. A detailed case history, physical examination and investigations like chest X-ray and Pulmonary Function Test, complete blood count, urine examination, and sputum examinations were done. After confirmation o f COPD as diagnosis, these patients were taken for the study.Lung function was estimated by spirometry in both the groups by calculating the ratio of forced expiratory volume in 1 sec and forced vital capacity FEV1/FVC < 70% was diagnosed as COPD.
All 50 patients of cae and controlgroup were examined for gingival and periodontal status by recording the following indices: oral hygiene index, plaque index(Silness and Loe1964),gingival index (Loe and Silness 1963), pocket probing depth and clinical attachment loss.Periodontal health was assessed by measuring (a) probing pocket depth (PPD) from the crest of the gingival margin to the base of periodontal pocket; (b) Mean clinical attachment loss (CAL) from cements enamel junction to the base of the periodontal pocket using William's graduated periodontal probe; and (c) Oral hygiene index (OHI, Greene and Vermilion; 1964),[18] which comprises debris index and calculus index.
Statistical Analysis
Data were summarized as Mean ± SD. Groups were compared by independent Students t test while discrete (categorical) observations were compared by Fisher’s exact test. The association of periodontal disease (CAL levels) with respiratory infections (FEV1/FVC ratio) was compared by one way analysis of variance (ANOVA) and the significance of mean difference between the groups (levels) was done by Tukey’s post hoc test. A two tailed (α=2) P<0.05 was considered statistically significant.
Results
Basic Characteristics
The basic characteristics viz. genders, age and smoking habits of two groups were summarized in [Table 1]. In both groups, the frequency (% age) of males was higher than females. The age of both controls and cases ranged from 40-65 yrs with mean (± SD) 56.60 ± 6.83 yrs and 55.80 ± 6.66 yrs, respectively with slightly higher being in controls. Similarly, in both groups, the frequency of non smokers was higher than the smokers. However, the frequency of smokers in cases (32.0%) was higher than the controls (16.0%). On comparing, all basic characteristics of two groups were found to be the same i.e. did not differed significantly (P>0.05). In other words, subjects of two groups were gender and age matched and also matched with the smoking habits.
[Image 1
Periodontal Parameters
The levels of different periodontal parameters viz. OHI, PI, GI, PPD, CAL and FEV1/FVC ratio of two groups were summarized in Table 2 and also shown graphically in Fig. 1. Table 2 and Fig. 1 both showed that the mean levels of OHI, OHI, PI, GI, PPD and CAL were significantly (P<0.001) higher in cases while FEV1/FVC ratio was significantly (P<0.001) lower as compared to controls.
 | Table 2: Periodontal Parameters Summary (Mean ± SD) Of Two Groups
 |
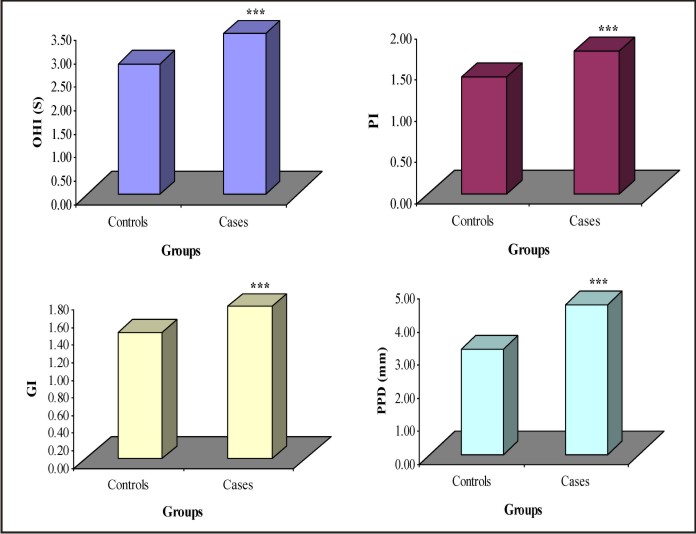 | Fig. 1: Bar Graphs Showing Mean Levels Of Different Periodontal Parameters Of Two Groups.
 |
Association between periodontal disease and respiratory infections
To see the association between periodontal disease and respiratory infections, the FEV1/FVC ratio was further average according to CAL levels and summarized in Table 3 and also shown graphically in Fig. 2. Table 3 and Fig. 2 both showed that as CAL levels increases mean FEV1/FVC ratio decreases. It suggests that there may be an inverse relation between CAL (periodontal disease) and FEV1/FVC ratio (respiratory infections). On comparing, the mean FEV1/FVC ratio at 4-5 mm and > 5 mm of CAL were found to be significantly (p<0.05 or p<0.001) different and lower as compared to CAL at < 3 mm and 3-4 mm. Further, the mean FEV1/FVC ratio at > 5 mm of CAL was also found to be significantly (p<0.05) different and lower as compared to CAL at 4-5 mm.
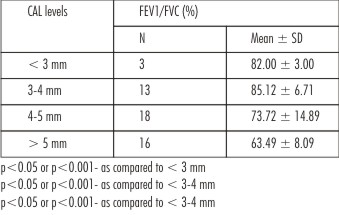 | Table 3: Association Between Periodontal Disease (Cal Levels) And Respiratory Infections (FEV1/FVC Ratio)
 |
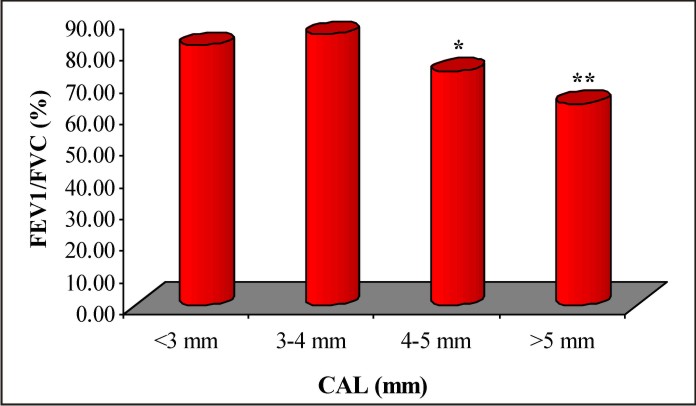 | Fig. 2: Bar Graph Showing Mean Fev1/Fvc Ratio According To Cal Levels.
 |
Discussion
Patients were demographically matched . Smoking status was evaluated in both groups .A significant difference was seen in CAL in control group but not in case group between smokers and non-smokers similar to the findings by V.Deo (2009).[19] The findings of present study suggest chronic periodontitis as a potential risk factor for COPD A significantly higher mean OHI , PI , GI, PPD and Clinical Attachment Loss was found in individuals with COPD (case group) compared to control group. Similar findings - Scannapieco et al (1998),[20] Gracia and Hayes et al(1998)[14]. Notwithstanding, Scannapieco and Ho[20] and Hayes et al. found a tendency toward diminished pulmonary function with increasing clinical attachment loss. In a retrospective longitudinal study Dental plaque can serve as a reservoir for potential respiratory pathogens. (Yuan A et al 1994).[21] Poor oral hygiene results in an increase in mass and complexity of dental plaque ,which may foster bacterial interactions between indigenous plaque bacteria (P.gingivalis, F. nucleatum) and acknowledged respiratory pathogens (P.aeruginosa, Klebsiellapneumoniae) which are shed into saliva. (F A Scannapieco1998).[14] As Clinical Attachment Loss increased, the mean FEV1/FVC (%) decreased - an inverse relation between CAL ( periodontal disease ) and FEV1/FVC% (COPD).Subjects with more clinical attachment loss had a higher prevalence of diminished lung function . Similar results by Scannapieco and Ho(1998).[21] In untreated periodontal disease cytokines produced by epithelial and connective tissue in response to these bacteria include IL-1α.IL-1β, IL-6, IL-8 and TNF-α Theyrecruite neutrophils to infiltrate airway parenchyma and release proteolytic enzymes and toxic oxygen radicals damage respiratory epithelium making it more susceptible to COPD (Travis et al1994). Oral hygiene maintenance by chemical and mechanical means leads to reduction in progression of COPD (Azrpazooh 2006).Smoking has also been reported to be a residual confounding factor in studies of associations between oral conditions and respiratory infections, given that this compromises the mucociliary barrier and phagocyte activity. Some studies have shown that the worse the periodontal condition, the greater the risk of chronic obstructive pulmonary disease among smokers [22],[23]. Good oral hygiene seems to diminish the levels of enzymes that degrade fibronectin, which originate in the dental biofilm or polymorphonuclear leukocytes found in saliva[24],[25]. DeRiso et al.testedoropharyngeal decontamination using 0.12% chlorhexidinedigluconate in patients who would be undergoing surgical procedures. They obtained a reduction in the nosocomial infection rate of 65%.[26]
Conclusion
Present analysis substantiates chronic periodontitis as a potential risk factor for periodontal disease.
References
1. Cullinan MP, Ford PJ, Seymour GJ .Periodontal disease and systemic health: current status. Aust Dent J 2005 ;54Suppl 1: S62–69.
2. Blaizot A, Vergnes JN, Nuwwareh S, Amar J, Sixou M .Periodontal diseases and cardiovascular events: meta-analysis of observational studies. Int Dent J2009 ;59: 197–209.
3. Preshaw PM, Alba AL, Herrera D, Jepsen S,Konstantinidis A. Periodontitis and diabetes: a two-way relationship. Diabetologia 2012; 55: 21–31.
4. Xiong X, Buekens P, Vastardis S, Yu SM . Periodontal disease and pregnancy outcomes: state-of-the-science. ObstetGynecolSurv 2007; 62: 605–15.
5. Megson E, Kapellas K, Bartold PM .Relationship between periodontal disease and osteoporosis.Int J Evid Based Health 2010;8: 129–39 .
6. Detert J, Pischon N, Burmester GR, ButtgereitF .The association between rheumatoid arthritis and periodontal disease. Arthritis Res Ther 2010; 12: 218.
7. Azarpazhooh A, Leake JL.Systematic review of the association between respiratory diseases and oral health. J Periodontol2006;77: 1465–1482.
8. Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease.AnnPeriodontol 2003 Dec;8(1):54-69.
9. Hill K, Goldstein RS, Guyatt GH,Blouin M, Tan WC. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ2010 ;182: 673–678.
10. Gomes-Filho IS, Passos JS, Seixas da Cruz S. Respiratory disease and the role of oral bacteria. J Oral Microbiol 2. ructive pulmonary disease. A systematic review. Ann Periodontol2010 ;8: 54–69.
11. Scannapieco FA. Role of oral bacteria in respiratory infection.J Periodontol. 1999;70:793-802.
12. Mojon P. Oral health and respiratory infection. J Can Dent Assoc. 2002;68:340-345
13. Scannapieco FA, Papandonatos GD, Dunford RG. Associations between oral conditions and respiratory disease in a national sample survey population.Ann Periodontol. 1998;3:251-256.
14. Hayes C, Sparrow D, Cohen M. The association between alveolar bone loss and pulmonary function: the VA Dental Longitudinal Study. Ann Periodontol. 1998;3:257-261.
15. Scannapieco FA, Ho AW. Potential associations between chronic respiratory disease and periodontal disease: analysis of National Health and Nutrition Examination Survey III. J Periodontol 2001;72:50-56.
16. Estes RJ, Meduri GU. The pathogenesis of ventilator-associated pneumonia: I. Mechanisms of bacterial transcolonization and airway inoculation. Intensive Care Med 1995;21:365-83.
17. Fatemi K, Banihashemrad S, Tovhidi M, HosseiniS . Evaluation of the Relationship Between Periodontal Disease and Chronic Obstructive Pulmonary Disease. J Mash Dent Sch 2009; 33: 214–16.
18. Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc 1964;68:7-13.
19. DeoV,BhongadeML,AnsariS,ChavanRS.Periodontitis as a potential risk factor for chronic obstructive pulmonary disease :A retrospective study. Indian J Den Res. 2009 Oct-Dec;20(4):466-70.
20. ScannapiecoFA,HoAW.Potential association between chronic respiratory disease and periodontal disease : analysis of National Health and Nutrition Examination Survey III .J Periodontol2001 Jan ,72 (1):50-6.
21. Oral hygiene,Periodontal health and chronic obstructive pulmonary disease exacerbations. Liu Z, Zhang W,Zhang J ,Zhou X,ZhangL,SongY,WangZ.JClinPeriodontol 2012Jan ;39 (1):45-52.
22. Loesche WJ, Syed SA, Stoll J. Trypsin-like activity in subgingival plaque. A diagnostic marker for spirochetes and periodontal disease.J Periodontol.1987;58:266–73
23. Beighton D, Radford JR, Naylor MN. Protease activity in gingival crevicular fluid from discrete periodontal sites in humans with periodontitis or gingivitis.Arch Oral Biol. 1990;35:329–35.
24. Garcia RI, Nunn ME, Vokonas PS. Epidemiologic associations between periodontal disease and chronic obstructive pulmonary disease. Ann Periodontol. 2001;6:71–7.
25. Wang Z, Zhou X, Zhang J, Zhang L, Song Y, Hu FB, Wang C. Periodontal health, oral health behaviours, and chronic obstructive pulmonary disease. J ClinPeriodontol.2009;36:750–5
26. DeRiso AJ, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidinegluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest.1996;109:1556–61
|